Expert Help Finding the Best Laser for Your Needs

TerraQuant / MR4
- Professional and Home Use
- Up to 50 Watt Peak Power
- Best Selling U.S. System

Avant Systems
- The Most Flexible 3b Laser
- Pulsed Sweep and CW
- IR/ Red / Violet
- Broad and Pinpoint

PowerMedic
- Handheld 1W-3W
- Hyperpulsing
- 810nm Danish Design
- Exceptional Quality
Vectra Genisys
- Pro Only Systems
- Optional Electrical Stim
- 670nm-950nm
- Up to 1.04 Watts Pulsed or CW

3B Scientific
- Multipoint Therapy
- 12 Simultaneous Points
- 660nm and 785nm
- Acupuncture /Trigger Point

ReGen Laser
- The State of Art
- Class 4
- Up to 6 wave
- Up to 104w
EVOLaser
- 9W-27W
- Single, Dual and Quad Wave
- Continuous and Pulsed
Apollo Lasers
- Handheld, Portable, and Desktop Versions
- Broad and Pinpoint
- 2-5 Watts Continuous

Used Systems
- Pro Only Systems
- Optional Electrical Stim
- 670nm-950nm
- Up to 1.04 Watts Pulsed or CW

ReGen Pod
- 7.6 kW Pulsed & CW
- Whole Body Pod
- Incredible Results
- The Absolute Best Pod

Guide to Laser
- Learn More

Compare Every Brand
- Learn More
Our store and training center is located at 921 Main Street in old-town Louisville, CO. We are one of the nation's only (if not THE only) walk-in brick-and-mortar specialty therapy laser store focused 100% on cold lasers and photobiomodulation. We work in all areas of cold laser development including independent research and we work with laser manufacturers and customers to help specify products that buyers want. What really makes us stand out is that we are independent of any one manufacturer so we can give you unbiased advice about which system should best meet your needs. We will beat anyone's price on a system but what really makes us standout is our service, support and customer dedication. For transparency, we partner with BioPhotonica, who builds the ultimate whole body light pod (the ReGen Pod), high intensity light panels (Regen Panels) and state-of-the-art class-4 lasers (ReGen Lasers).
We sell a wide range of cold lasers (class 1 to class 4) so we can help you find the best laser system for your needs and we won't tell you that one laser system is always the best because it is not true. Each product we sell has an sweet spot. The best system for you depends on your needs and we understand that. All of our products have a 30 day money back guarantee* so we only sell system with a prove track record of delivering results. If you want to dig into research or watch some video training, our BioPhotonica education site is Laser-Therapy.US or you can find it in the apple and android app stores
In addition to having a minimum performance level that we will sell, we also discourage the average user from wasting their money buying more power than they really need. Underpowered system can be too slow that people give up before the see results but they still work on many smaller applications. Systems over 40 watts are perfect for busy practices but can be a waste of money for some home users. We feel that the lower-powered and higher-powered systems are great for some applications but they are not everyone's cup of tea.
We sell Avant, EVOlaser, Apollo, Chattanooga Vectra Genisys, Laserex, PowerMedic, Biophotonica, Multiradiance (Terraquant), Microlight, and 3B Scientific. For anyone looking for a truly "cold" system, we often use and highly suggest the Multiradiance or Microlight systems. In the past, we have sold several other leading brands of cold lasers so we know all the major brands of therapy laser so we can help you learn more about all your options, the technology and the best applications for each device (including ones we do not sell). If you browse our laser buyer's guides, you can see specific recommendations based on your application including treatment of horses and companion pets. We also make different recommendations based on broad coverage therapy versus laser acupuncture and trigger point therapy. If your goal is unattended laser therapy, this significant reduces your options but there are still some good choices. In the field of dentistry, we offer several good dental lasers. We have different recommendations for professional therapy lasers versus home laser systems but our cold laser guide shows all the most popular and well respected lasers on the market so it is a great article to compare options. We can also help you find a quality laser to meet your budget requirements. We sell to both doctors and consumers. Feel free to call us at 1-800-388-0850 or use the chat option to get help.
At ColdLasers.Org we present information and specifications of many different professional grade lasers. Unlike exclusive sales people, who must sell you their product even if it is not a good fit, we feel than an educated consumer is a happier customer and we want buyers to understand all their options and their regional regulations and help them to get the best equipment for their needs even if they buy a product we don't sell. All these products have a good reputation with professionals and practitioners so they are all worth investigating. We do not promote products that have extraordinary or magical claims that are not based on traditional photobiomodulation. We avoid products marketed based on pseudoscience like scalar waves, quantum waves, soliton waves and zero point energy (1). We like to stick to science based products and we don't see any reason to make mystical claims when the science is so solid. We like to stick to the facts and specs. There are some people selling very questionable products including stickers that magically boost the power output of a lasers so buyers must beware.
The core of laser therapy is based on using lasers to drive a specific wavelength of light energy into the cells to directly stimulate the mitochondria (a light sensitive component inside every cell) to convert glucose into ATP (adenosine triphosphate). ATP is considered by biologists to be the “energy currency of life”. It is the high-energy molecule that stores the energy our bodies need to do just about everything including cellular motion, cellular division, protein synthesis and repair.Other Research shows laser therapy results in the manipulation of inducible nitric oxide synthase (iNOS) activity, suppression of inflammatory cytokines such as TNF-alpha, IL-1beta, IL-6 and IL-8, upregulation of growth factor production such as PDGF, IGF-1, NGF and FGF-2, alteration of mitochondrial membrane potential due to chromophores found in the mitochondrial respiratory chain as reviewed in, stimulation of protein kinase C (PKC) activation, manipulation of NF-κB activation, direct bacteriotoxic effect mediated by induction of reactive oxygen species (ROS), modification of extracellular matrix components, inhibition of apoptosis, stimulation of mast cell degranulation, and upregulation of heat shock proteins [1]. |
The Main 4 Theories of Cold Laser Effectiveness
If you analyze the entire cold laser market, you will find that there are basically 3 different philosophies of what makes a laser great.
- Put the optimum energy (Dosage measured joules) into the tissue: This is the core technology of photobiostimulation. ColdLasers.Org agrees that proper dosage is the core technology and probably has the highest correlation to efficacy.
- Pulse the laser to get an additional reaction from tissue: Pulsing adds an extra dimension to lasers and allows lasers to deliver higher peak energy levels while still being safe. In some applications, the pulsing frequency is of upmost importance. Pulsing can also make it harder for the body to become resistant to the therapy so it is more important for longer term treatment plans. This feature is great to tweak the laser for slightly better results but it is over-hyped by some manufacturers. Systems that only focus on pulsing often provide inconsistent results and there are several scam laser companies that claim unrealistic pulsing properties.
- Use different wavelengths to simulate different reactions: Different wavelengths interact with the body in a different ways. Every manufacturers says they have the best wavelength ranging from 1350nm to 400 nm. 90% of professional therapy lasers operate in the 620nm to 980nm range. Research shows there is sweet spot at 800 to 810 nm provide the best efficiency at driving the energy deep and still getting a photo-chemical reaction. We call 808/810nm a primary wavelength. If you only have one, this is the one you want. For non-structural issues, there are other options like 600 to 660nm for superficial issues. If your primary focus is pain control and increasing circulation, then 980nm is mostly converted to deep heat near the surface so it is the best option. If you have time to do research, there are lots of publication to show what is the best wavelength for laser therapy.
- Protocols: Results are only as good as the protocols (treatment plans). The best practice is to adjust for the laser specs, condition, skin color, patient size, cronicity, and pain level. Better protocols will also allow custom pulsing to acheive a secondary goal other than ATP production.
After years of working with tens of thousands of people, it is obvious that all these factors are part of the equation. The therapy laser market is mostly established ethical companies but there are a few sketchy hyper-marketing companies. When comparing lasers, it is wise to buy based on specifications more than the marketing claims.
Dosage
The single most important factor in successful laser therapy is getting the right dosage. Special pulsing and wavelengths can help tweak the laser for maximum results but dosage of the right wavelength is the key to success. Just like we see in the pharmaceutical industry, delivering the correct dosage is the difference between success and failure. If the dosage is too little, nothing happens. If the dosage is too high, we do not worry about a life threatening overdose but we waste a lot of time and money and sometimes get less positive results. Dosage for laser therapy is measured in total joules o r joules/cm2 at the depth of the damaged area. Larger treatment areas and deeper areas require more dosage. When Turner and Hode analyzing all the unsuccessful studies on LLLT that people use to discredit laser therapy, they found in every case that the dosage was too low that they should not have shown positive results. Throughout this website, you will see information about how different conditions require different dosages and how different lasers are best suited for different applications.
Unfortunately, there is a lot of misinformation about cold lasers on the web. Low power laser manufacturers publish studies and articles trashing higher power laser manufacturer and vise versa. When we first started doing research, the information was incredibly confusing and convoluted. We built this site to help people clear through the fog of conflicting claims. We don't bash any science-based systems because we know each system has a niche but our general rules are "if it looks like a laser pointer, it probably is a laser pointer".
Pulsing & Continuous Wave (CW) Output Lasers
Lasers can be either continuous wave or pulsing output . Continuous wave means that the laser is turned on 100% of the time during the treatment and a pulsing laser is turned on and off very quickly during the treatment. Pulsing is defined by 2 variables:
- Pulsing frequency measured in Hz (how many times it turns on in one second)
- Duty cycle (measured as a percentage of ON time to total time).
There is little consensus in the laser community about what is the best pulsing frequency for laser therapy but research does show that pulsing is best for most applications with the exception of nervous system tissue damage where CW is best. The average pulsing setup is 25Hz to 500Hz and a 50% duty cycle. "Super-pulsing" lasers are much lower duty cycle but they are very high peak power. Most cheaper lasers are continuous wave output only.
Wavelengths Used in Laser Therapy
In general, each wavelength interacts with the cells in your body in a unique way. If you want to read more about the advantages and disadvantages of each wavelength, read our article on "wavelengths used in the laser therapy". Some people say that higher wavelengths are shorter so they can go deeper but the light absorption curve shows this is not true because some wavelengths are rapidly absorbed by some molecules.
760nm - 850nm: This wavelength (especially 808/810nm) is best for increasing cytochrome C oxidase and Adenosine Triphosphate (ATP). It is our recommendation for anyone seeking the best long-term benefit. It is offered by every single manufacturer we sell because it provides the best combination of depth of penetration and photo-chemical reaction. If you only buy one wavelength, 808/810nm is the one.
600nm - 680nm: Best for more superficial therapies and absorption by the blood. This wavelength is the better for treating abrasions, bruses, superficial cuts and dermatitis. Erchonia™, Aura™ and Avant™ are the main manufacturers who put an emphasis on this wavelength but many other manufacturers uses it as a supplemental wavelength.
900nm - 910nm: This wavelength is another widely popular option. It best at interacting with hemiglobin, water, melanin and Cytochrome C oxidase. This wavelength is for "increasing oxygenation in the blood". At this wavelength, much of the emphasis is on the pulsing technology.
920nm - 980nm: From the tissue absorption graph, we can see that these wavelengths have lower efficiency at providing deep photo-chemical reaction since much of the energy is absorbed by water in the tissue and is converted into heat.
Over the years, this range has become "the standard" for pain clinics and neuropathy centers because the much of the energy is absorbed near the surface where we have pain sensors so it is a good secondary choice if you want maximum pain and inflammation control.
1064nm: This wavelength is absorbed by the primary Chromophores and it has a lower depth of penetration so it is also a good 2nd or 3rd wavelength for maximum pain control.
380 to 495nm (Violet and Blue): These wavelengths are used for anti-bacterial and anti-viral applications. Violet is the a wavelength used by Erchonia™ (405nm) and Avant (405nm). Klaser (445nm) and Biophotonica (450nm).
Under 380nm (Ultraviolet): - UV is not used in therapy as it can disrupt the DNA.
500 to 760nm: There are some fringe lasers using yellow and green diodes but these wavelengths are not backed by research yet and they are not used by any of the industry-leading professional therapy laser companies.
The majority of the therapy lasers in the world are either 800-860nm range or at 905-910nm. 905nm was made popular by Multiradiance (who makes the TerraQuant and MR4 lasers) and they have sold more systems than anyone else in the industry. There is some good research that shows that 800-860nm has the best balance of properties for a single wavelength laser if your goal is treating deep damaged tissue. 600-660nm is best for burns, lymph system treatments, acupuncture, superficial and complex issues where there is not a defined treatment area. 980nm is the most common for higher levels of pain control and 905nm is best for home system because they are a good balance of safety and efficacy
3 Types of Applicators
There are 3 basic styles for treatment using cold lasers, pinpoint treatments (acupuncture, laserpuncture or trigger point), broad therapy and hands free therapy. Each treatment style has a different goal and different equipment requirements. In many cases, the same condition can be treated with 3 totally different strategies of cold laser therapy.
| Pinpoint | Direct Treatment | Hands Free | |
| Strategy | Treatment of meridians, trigger points, acupoints, small body parts or lymph system. | Direct treatment of the damaged tissue. | The emitter is held at a distance so that the entire area can be treated at once. |
| Treatment Area | 2 mm2 to 20 mm2 | 60 mm2 to 250 mm2 | 60 mm2 to 6000 mm2 |
Laser Trigger Point And Acupuncture Therapy
In laser trigger point therapy, the cold laser is used similarly to acupuncture or acupressure to trigger a reaction from the body by stimulating an acupoint. In this case, a focused low level laser beam is used to concentrate all the energy from the cold laser into a very small area. Cold lasers are often compared to "acupuncture with a laser beams". In LLL laser puncture treatments, the laser beam is use to trigger the body's acupoints without the fear or pain of needles. The maximum safe power for these concentrated energy systems is 500mW. If you read our analysis of the dosage numbers, you will see that 5mW laser pointers will never achieve a adequate dosage in a reasonable time.
Direct Treatment Therapy
In many cases, if a practitioner may not be targeting a trigger point, they will choose to use a cold laser to energize a larger area of damaged tissue in the body. In this case, a cold laser with a broad focus (larger than the size of a dime) and the correct wavelength are used to penetrate the deep tissue with photons direct energize the area. This is typically done by moving the laser and skin contact is preferred if the area will allow it. These large emitters can cover areas up to 4.6 inches square. The larger treatment area increase the chances of stimulating any damaged "hot spots". Larger emitters can also reduce the treatment time and provide a more even energy distribution over a larger treatment area. The size of the treatment area and the depth of the treatment area often dictates a minimum power requirement. The following table shows sample dosages for a small/shallow and large/deep injury and calculates the treatment times to get the same energy concentration evenly distributed over the treatment area at the desired depth. This table illustrates how treatment area and depth increase the required dosage and then power determines the treatment time for that condition.
| Area (cm2) |
Depth (cm) |
Dosage |
Time @ |
Time @ |
Time @ |
|---|---|---|---|---|---|---|
Arthritic |
6 |
0 - 2 |
10 - 300 |
10 - 50 mins |
1 - 5 mins |
<60 seconds |
LB Pain |
77 |
2 - 6 |
300 - 10,000 |
.8 - 27 hours |
5 - 166 mins |
1 - 16 mins |
| Diabetic Neuropathy | 1200 | 0 - 2 | 1000 - 26,000 | 2.6 - 72 hours | 16 - 433 min | 1 - 43 mins |
This table illustrated why larger, deeper and more complex conditions require more powerful lasers to deliver an appropriate dosage in a reasonable amount of time. Lower dosages will still help but often the higher dosages shown here will provide more consistent results.
Hand Free
The third option is hand free. With these systems, the emitter is fixed and treats the entire area at one time. There is some loss as the energy travels through the air so treatment times must be increased versus direct contact but this system can be used for unattended therapy so there is no cost associated with having someone hold the laser during the treatment. For busy practices or for areas that can not be reached easily (like the mid back) by home users, hands free is the best option. Hands free system come in class 1 through 4.
FDA Clearance of Cold Therapy Lasers
Over the years, we have tried to summarize a lot of information about different cold laser technologies and styles of cold laser therapy. The FDA cleared applications for almost all cold lasers (sometimes called low level lasers, soft lasers or therapy lasers) are pain control, inflammation reduction and increased blood circulation. Some manufacturers have extended these claims to include accelerated healing but that is not an FDA cleared claim. There are over 4000 positive published studies, dozens of books and hundreds of videos showing the efficacy of cold lasers to treat a variety of applications. We have sold systems to several branches of the U.S military, the Veterans Administration, the US Indian Health Services and many medical doctors (MDs) but acceptance of the technology is still held back by insurance companies who falsely think they are saving money paying for a lifetime of prescriptions when a cource of laser-therapy would solve the problem. Even with over one million lasers current in the hands of professionals and home users, many people still question the efficacy of cold lasers but the momentum of laser therapy and PBMT is growing.
Unlike hot medical lasers, which are widely used to cut and cauterize tissue, Low Level Lasers (LLLs) or cold lasers penetrates the surface of the skin with minimal heating effect. We sell several different class 4 lasers that will warm the treatment area but these are still in the same category as cold lasers because they are not hot lasers.
In most cases, cold laser therapy is considered an alternative therapy like acupuncture, message therapy, chiropractic, herbal medicine, message, and physical therapy because it does not require surgery or a life-long prescription to drugs. Practitioners have supported treatment options like ultrasound and electrical stimulation for years and now there is a lot of interest in cold lasers as a supplement to their practices. Therapy lasers have provided relief for hundreds of millions of patients and they work perfectly in combination with many other traditional and alternative modalities.
Currently, there are over 50 different cold laser manufacturers that have products that have been cleared by the FDA for various types of treatments. Cold lasers have been used around the world for over 30 years and have been in use in the US since 2001 when Microlight got the first FDA clearance. Low level laser therapy or soft laser therapy has been proven completely safe and effective in thousands of worldwide studies and there are lots of great books to help users get the most out of their laser.
The power level of therapeutic cold lasers ranges from 5 milliwatts (0.005 watts) to 102,000 milliwatts (102W). This energy can be created using one laser diode or an array of laser diode. In low-end therapy devices, it is generated using LEDs or SLDs. In many systems, an array of laser beams can increase the total power output with less risk of heating since the energy is evenly distributed over a large area. Since finding the optimum treatment spot deep inside the tissue is somewhat of an "educated guess", it can be very useful to have a larger aperture on the treatment probe. This increases the probability of getting the photons to the problem area and larger emitters also also help increase the energy in the area surrounding the main target area. Emitters that use a combination of different wavelengths also have a better chance of treating tissue at different depths since different wavelength have different absorption rates and can interact with the cells in different ways.
The average cold laser therapy session cost from $30 to $200. The average cold laser costs from $2000 - $30,000 so the return on investment (ROI) on purchasing a cold laser can be as little as 3 months.
Because cold lasers help activate human and animal tissue at a cellular level, cold lasers can be used for cervical (neck) pain, lumbar (low back) pain, wrist pain and injuries (carpal tunnel), elbow and joint pain and injuries, lower extremities pain, foot and ankle pain, joint pain and knee injuries, neuropathy, accelerating recovery after surgery and hundreds of other applications. You can use the research tool at laser-therapy.us to search for published scientific studies, books, videos and other resources.
Laser Power Requirements
The dosage of laser energy is measured in joules [J] and is equal to the power in watts of the laser multiplied by the treatment time. A 1 watt continuous wave laser delivers 1 Joule/sec at the surface. For a pulsed laser, the laser is off part of the time so the delivered power is derated by the duty-cycle (ratio of ON to TOTAL time). A laser with more power, measured in milliwatts (mW) or watts (W) or a higher duty-cycle laser can deliver more energy in less time. Delivering joules is where the bulk of the work is done when treating patients. The class of the laser (1 through 4) is largely controlled by the power level from each output beam because they are somewhat proportional to the potential for damage. If you want to dig into the importance of power, read our article about " Laser therapy power requirements".
Why Some Lasers are Ineffective
Many laser companies make a huge mistake on their protocols. They will use the WALT recommended target energy (which is lower than most US manufacturers recommend) "at depth" (4-8 joules) as the requirement for the output of the laser. This is a huge mistake. At a depth of 1 inch (2.54 cm), up to 80% of the energy has already been absorbed on its way to the damaged area so you must increase the dosage out of the laser to accommodate for the lost energy. That means that to get 8 joules/cm2 at depth can requires 26 joules/cm2 at the surface. Since most system have a treatment are of about 1 inch2 (1 inch2 = 6.4 cm2), the laser should output 167 joule for a single point. If you are moving the laser around, you can easily see why many lasers are underpowered. If you want 8 joules/cm2 at depth over a 3 x 5 you need 2505 joules out of the laser. You can use the table below to see the treatment times for a range of dosage requirement.
How Much Power is Enough?
Too Little Power: In our summary of laser power requirements, you can see that there are many inexpensive over-the-counter lasers might help with small issues but are drastically underpowered for many cases. If we look at the theoretical treatment times based on the power of the laser, it is easy to see that these products will never reach the typical dosages that you would get in a doctor's office or clinic. With a 1mW system, it takes 5.7 days to achieve 500 joules at the surface of the skin and 500 joules over a large area (like a back) or into a deep area (like a hip) is not delivering any significant energy. These low-powered lasers are often more powerful than LED therapy systems but they are massively underpowered when compared to professional systems. We do not sell any systems without a proven track record because these systems do not provide consistent efficacy*.
| Target Energy @ Surface | 50 J |
500 J |
1500 J |
5000 J |
15,000 J | |
| Laser Power | Rate (J/min) | Treatment Time |
||||
| 5 mw | 0.3 | 2.7 hours | 1.1 days | 3.4 days | 11 days | 33 days |
| 100 mw | 6 | 8 minutes | 83 minutes | 4.2 hours | 13 hours | 39 hours |
| 500 mw | 30 | 1.6 minutes | 16 minutes | 50 minutes | 2.7 hours | 8.1 hours |
| 1W | 60 | 50 seconds | 8.3 minutes | 25 minutes | 83 minutes | 4.1 hours |
| 5W | 240 | 10 seconds | 1.6 minutes | 6.5 minutes | 4 minutes | 12 minutes |
| 10W | 600 | 5 seconds | 50 seconds | 3 minutes | 8 minutes | 24 minutes |
| 30W | 1800 | 2 seconds | 12 seconds | 1 minutes | 2.6 minutes | 7.8 minutes |
| 60W | 3600 | < 1 second | 12 second | 30 seconds | 1.5 minutes | 4.5 minutes |
Too Much Power: As long as you can keep the skin temperature in the desired range (warm but not hot), there is no such thing as having too much power. It is possible to spend too much money over-buying power that you don't need but that is why we work with our customers prepurchase. Higher power lasers buy you shorter treatment times and/or higher dosages in the same treatment time. Higher dosages often provide a wow factor that you rarely get from other modalities. In general, the more hours per day you spend using the laser, the more power you want. If you double the power of the laser, you halve the treatment time so that really add up if you are using the laser alot. In summary, higher power equipment is the best option for anyone wanting shorter treatment times and faster results.
It is commonly accepted that photobiomodulation is biphasic, meaning that there is a point where excess power will decrease the benefit. Many of the lower power laser manufacturers claim that you can put too many joules into the tissue and inhibit the cells, not stimulate them. Based on the famous Arndt-Shultz diagram, they claim that it is easy to overdose tissue. It is common sense that there must be some point of diminishing return but no one really knows if we are even close to reaching that point with higher power systems. In one of his videos, Doctor Hamblin says that over 645 joules/in2 (100 joules/cm2) is "probably" too much. If we are treating a deep problem where 80% of the energy is lost on the way to the problem or a large problem, it is easy to see that a total dosage of 20,000 joules could still be below this threshold.
Emitter Coverage Area
The larger the emitter coverage area, the more consistent the energy distribution and the cooler the tissue will stay while absorbing higher dosages. A larger emitter will is more likely to correctly illuminate a damaged area and since many treatments require moving the probe around a damaged area, a larger emitter can make the theray safer and more consistent. Many protocols use a combination of static treatments surrounded by a sweeping treatment around the entire problem area. Products with larger treatment areas are safer AND easier use. If you have a large bruise (damaged tissue) the size of your hip and you needed to "color in" the area with ink, you would want to use a big fat marker and not a ball point pen. When you are treating damaged tissue, you are essentially "painting" the tissue with light. In many cases, you might not even know the exact area that needs treatment the most. By using a larger emitter, you increase the odds that you will illuminate the critical spot. With the exception of trigger point and acupoint therapy, which is typically done by a highly trained professionals, the larger the emitter the better. Pinpoint emitters like the ones listed on our acupuncture page are not optimized for treating broad tissue damage and should only be used if there is no other options.
Treatment Times
Treatment times typically range from 7 seconds to 40 minutes with most therapies averaging 12 minutes. Most therapies require 1 to 6 treatment locations and an average course is 12 to 24 treatments. For those with 2 bad knees or wide spread arthritis, the total treatment time can really add up. For home use, treatment times are typically not an issue since users can often perform the therapy while relaxing or watching TV. In a doctor's office, treatment times can be a big issue. It is a common practice in the cold laser market for low-power laser manufactures to specify shorter-than-optimum treatment times because they know that no one would buy a product if they knew it would take 24 hours a day of therapy to reach a reasonable energy level in the deep tissue. The result is that patients never get even close to the a reasonable dosage and so they often think that LLLT is not effective. This is why we like to talk about the key specifications of lasers like wavelengths and power levels. We try to stay away from the marketing hype.
Protocols & Training
A complete protocol (treatment plan) library is key for new cold lasers owners to get the optimum energy into the treatment area without guesswork. Some manufacturers have created "cookbook" style protocol manuals or internal libraries. Others give general guidelines and allow professionals to develop their own optimum treatment plans based on experience. This is a simple task for some hands-on practitioners like chiropractors and acupuncturists. To make sure that everyone who purchases a laser from ColdLasers.Org is successful, we include a free membership in Laser-Therapy.US. With over 250 dynamically-created pictorial protocols (for humans, horses and dogs) and a therapy timer for effortless therapy sessions, it is one of the best sources for learning more about how to use a laser on a wide variety of problems
Depth of Penetration and Power
It seems like everything associated with cold lasers has some kind of controversy and that includes depth of penetration and its relationship with laser power. Some say that higher power lasers push the energy deeper but this is not technically correct. All lasers of a similar wavelength are losing energy at the same rate as it travels through the tissue. That means that for any treatment depth, all lasers of a similar wavelength lose the same percentage of energy but the power level controls how fast the energy builds up at that depth. To illustrate, let's compare a 1mW laser (like the ones you can buy on Amazon) and professional 10,000mW laser in treating a small deep tissue problem like a torn meniscus. At a depth of 2 cm, 84% of the energy is gone for both systems. Even with 84% of the energy gone, a 10,000mW system is putting in 2 joules/second into the tissue at depth so the system can reach a recommended dosage of 4 to 12 joules/cm2 in less than 6 seconds (for 1 cm2). The LED or 1mW laser system is pushing .00016 joules/second so it will take over 2 hours to treat a single point (1 cm2). Treating a larger area will take days. Not everyone will agree with this concept but it is more technically correct to say that higher power systems push faster not deeper.
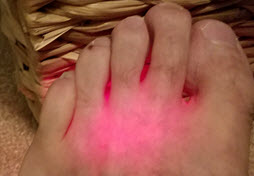 Above, we talked about how 800-860nm laser (which is invisible to the naked eye) have the best depth of penetration and 600-660nm has a much shallower depth of penetrations because it is absorbed by blood and melanin. So what is the actual depth? The RATS (reflection, absorption, transmission and scatter) curve for any wavelength slowly approaches zero but never does so technically manufacturers can say any depth they want. In practice we see that a shallow depth of penetration wavelength like 637nm has a depth of more than 1 inch. We can see this in the unedited image to the left. This image shows excess RED light coming out of the top a foot when the laser is applied to the bottom. In this case we were using a 1000mW Avant RED laser and the thickness of the foot is about 2.5 cm so RED has a depth of penetration of more than 1 inch. It is also interesting that we can also see less light where there are blood vessels because they has absorbed more of the energy. If we are working with a laser in the 800 to 860nm range, we should expect to see 16% of the energy still available at a depth of 2 cm and some energy is still being transmitted at 3 to 5 cm. Beyond this depth, we still see chemical migration from the photobiomodulation so it is best not to get too abscessed with depth of penetration.
Above, we talked about how 800-860nm laser (which is invisible to the naked eye) have the best depth of penetration and 600-660nm has a much shallower depth of penetrations because it is absorbed by blood and melanin. So what is the actual depth? The RATS (reflection, absorption, transmission and scatter) curve for any wavelength slowly approaches zero but never does so technically manufacturers can say any depth they want. In practice we see that a shallow depth of penetration wavelength like 637nm has a depth of more than 1 inch. We can see this in the unedited image to the left. This image shows excess RED light coming out of the top a foot when the laser is applied to the bottom. In this case we were using a 1000mW Avant RED laser and the thickness of the foot is about 2.5 cm so RED has a depth of penetration of more than 1 inch. It is also interesting that we can also see less light where there are blood vessels because they has absorbed more of the energy. If we are working with a laser in the 800 to 860nm range, we should expect to see 16% of the energy still available at a depth of 2 cm and some energy is still being transmitted at 3 to 5 cm. Beyond this depth, we still see chemical migration from the photobiomodulation so it is best not to get too abscessed with depth of penetration.
Why do we call it Cold Laser?
In general, because our goal is to keep the skin temperature as "cold" as possible while appying the dosage. There is some confusion about what to call the equipment that is described on this site. We also like the name therapy lasers but some people would consider skin care lasers as therapy lasers. Probably the most accurate is photobiomodulation (or PBM) lasert but this name doesn't seem to stick with the average person. In some publications, you may see the abbreviation LLLT for Low Level Laser Therapy. Most class-4 lasers warm the skin during therapy so the name cold laser is not a that accurate either. There is really no perfect description for the wide range to therapeutic, veterinary and equine lasers so we just call them all cold lasers.
Professional And Home Use Systems
Cold laser are typically sold to practitioners but in most states there are no legal limitations for the use of cold lasers in the home. Selecting and learning how to use a laser can get complicated but when you buy a system from ColdLasers.Org, we will make sure that you get what you need.
Classes of Cold Lasers
All lasers, hot, warm and cold, are given a classification according to the international specification; IEC 60825. The more potential for eye damage, the higher the classification with class 4 lasers (typically over 500mW CW per beam) have the greatest potential for eye damage and class 1 have no potential for eye damage but are still a laser. Power level is a key factor in the application of cold laser therapy but this does not mean that higher class lasers are always better, it does typically mean that they are:
- More expensive
- Can deliver higher dosages for better results
Many higher power lasers often use optics to diverge the beam (typically 10 - 30 degrees). This creates a larger treatment area. By spreading the energy over a larger area, the product becomes easier to use, provides for more even energy distribution and it safer to the eyes.
Manufacturers can use multiple laser beams in an array to get to higher total power levels while reducing eye damage potential. Using this technique, it is possible to build a 18-watt class 3b laser that would deliver energy very quickly but have lower risk of eye damage.
We can help you find a balance between power, treatment time, cost and safety.
Pain Control versus Healing
If we look at all the claims made made by all the manufacturers, the general consensus is that higher dosages provide superior pain control but recovery is based on the accumulated dosage and not the one time dosage. This means that many lower dose therapies can achieve the same thing as a few higher dosages. Since many patients will give up on the therapy if they do not feel a noticeable improvement in the first few sessions, many practitioners start out treating with higher doses to make sure they notice a change. Some patients may prefer immediate pain relief as the highest priority and others may be patient enough to stick with the plan long enough to get a permanent long-term improvement. Since long-term results can takes weeks or months of therapy, it is best to develop a reasonable expectation at the beginning of the therapy.
Potential for Heating and Tissue Damage
There is a lot of confusion about tissue heating and the potential of therapeutic lasers to do tissue damage from excessive heating. It can and does happen but much of the risk is fear-based marketing generated by lower power laser manufacturers as a reason to buy their product. The risk of tissue heating with a class 1, 2 or 3 FDA cleared therapeutic laser is extremely low. You also don't need to worry about significant heating with a class 4 if it is used according to the laser manufacturer's recommendations. For divergent (energy is coming out of the emitter in the shape of a cone) lasers below about 4,000mW (4W), there is an extremely low chance of significant heating for most skin types. It is unclear if this true for some of the non-FDA-cleared cheap foreign built lasers on Ebay and other discount sites. Most class 4 laser with a power of 10 watts or more require that the emitter is kept moving when the system is on but you typically need to keep that emitter moving anyway since most treatment areas are larger than the emitter size.
Heating potential is increased if :
- The laser is collimated (not divergent)
- And the laser is set to continuous wave (not pulsed)
- And the power level is set to more than 4 watts CW (not pulsed)
- And the laser is not moving
- And the wavelength of the system is 980nm or higher
- Or the patient has very dark skin, birth marks or tatoos
It is very rare that anyone will do enough things wrong (static, CW max-power collimated treatment) to have a problem. For most therapies, you want to cover an area of 4 to 40 times the diameter of the emitter so you need to move the laser anyway. With higher power class 4 lasers, you simply have 2 reasons to move the laser; better efficacy and safety. Many practitioners use heat lamps with power levels up to 250 watts in their practice. If used improperly, heat lamps have more potential for burning. Just like with a heat lamp, you must check with the patient and make sure they are comfortable with their level of warming.
In exchange for this potential risk, you get the opportunity to blow patients away with almost instantaneous results. Since a 10-watt system is delivering 600 joules per minute, you simply keep it moving for about 4 minutes and you have 2400 joules into the total treatment area. That level of energy will typically produce much stronger response that you get with a lower power laser.
For many practitioners and users with serious problems, it's hard to deny that the option to deliver high dosages when appropriate is worth the slight risk of heating the tissue. If you want instantaneous pain relief, there is no substitute for power. If you really want the maximum wow factor (where patients walk away feeling totally different), a collimated class 4 laser is like no other. The real problem is that most class 4 systems are 980nm. 980nm is the best wavelength for heating the water in tissue. That is why they make great hot surgical lasers (just search for 980nm surgical lasers) and bad therapy lasers (much of the energy is lost in heating and not cellular interaction).
Insurance
Billing for the Service: Some cold laser therapies qualify for insurance reimbursement using a standard cold lasers CPT code. The most common is 97026 (infrared therapy). Cold laser therapy performed by a licensed practitioner typically can be paid for using an HSA account.
Buying a Laser: The purchase of cold laser devices may be covered by some insurance plans using the HCPCS code E1399 (generic durable medical device). In worst case, it is possible to force an insurance company to pay for a laser through the court system. Here is an article written by a customer who successfully forced her insurance company to pay for a home laser but it took about 1 year in the courts.
- If you want to see where our information comes from or you want to do your own research, try our cold laser therapy research tool.
- If you are looking for help navigating regulations, we have a summary of laser therapy regulations.
- If you are looking for video training on laser therapy, you can use our free LLLT video training program.
- If you are looking for a doctor in your area that does laser therapy, you can use our directory of doctors who offer laser therapy.
- If you want to see some of the protocols included in our protocol library you can visit our state-of-the-art cold laser protocol library.
Download our "Light & Laser Therapy" App from the Apple or Android Store. Research and Video training are free and you get access to all the protocol when you buy a system. |
|
 Apple iOS |
 Android |
Why buy from ColdLasers.Org? We provide the best training, service and support in the industry.